
So we can add/subtract in chemical equations, but its can't just simply make mathematical sense, it also has to make sense in a chemistry context. (3) Select a point value to view scoring criteria, solutions.
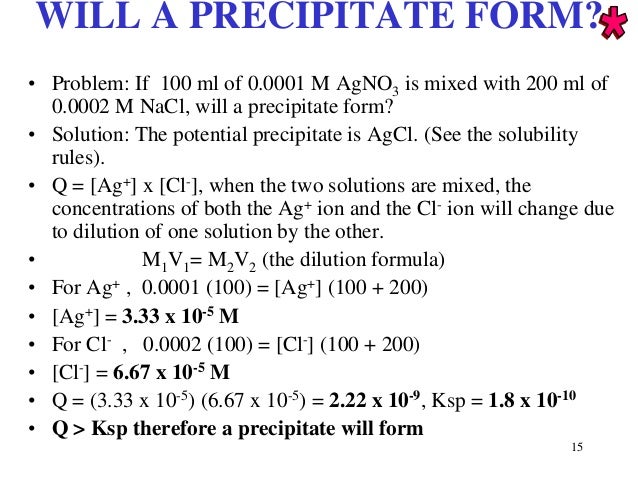
So it's not much that we're adding chemicals rather we're discovering the actual reaction occurring. In acid/base reaction its common for the H+, OH-, and H2O to be the only species left in a net ionic equation after all the other spectator ions have been eliminated. However if it's an aqueous solution, these added chemicals are technically always present in the reaction solution and what we're actually doing is recognizing that some of them are actually part of the redox reaction. A solid precipitate isnt the only thing you look for in net ionic equations, you also look for neutral covalent compounds like water forming. Now in this case where we're adding chemicals to the equation it may just seem like we're adding chemicals out of convenience to make the math work out. (c) Calculate the molarity of the original CuSO: 4: solution. In redox reaction it's common to add water, H+, and OH- ion to the equations when balancing them. Moles Of Precipitate Calculator Determine the number of moles of each ion left in the. So since they're not participating in the reaction, subtract them is allowed because it doesn't affect the reaction if they're absent from the equation. Also, 'precipitate' is the name given to the solid that is formed as a result of a precipitation reaction. If you get stuck, click on the equation to get a step-by-step solution. So these are ions which are present in the reaction solution, but don't really participate in the actual reaction (they don't change as a product compared to when they were a reactant). Updated on JanuIn chemistry, to precipitate is to form an insoluble compound either by reacting two salts or by changing the temperature to affect the solubility of the compound. Use the calculator below to balance chemical equations and determine the type of. For ionic equations like these it's possible for us to eliminate, essentially subtract out, spectator ions from an equation. Mathematically it's completely acceptable to do so, however we have to consider the actual chemical makeup of our reaction if we do so. This reaction is represented by the molecular equation below. So we might predict that a non-polar solvent that doesn't dissolve salts would be a bad solvent for a double replacement reaction.If we could zoom in on the contents of the reaction beaker, though, we wouldn't find actual molecules of AgNO 3 \text_4(aq) Na 2 SO 4 ( a q ) start text, N, a, end text, start subscript, 2, end subscript, start text, S, O, end text, start subscript, 4, end subscript, left parenthesis, a, q, right parenthesis.
#Solution precipitate calculator free
The more you know about how the reaction occurs, and the more you know about the properties of different solvents (like their polarity), the more educated of a guess you can make! For example, in double replacement reactions, we know that the solubility of the reactants is important because we need free ions around. In general, it's tricky to predict for any random reaction what medium it might need. The solvent and soluble components of the reaction are called the supernatant or supernate. Water is a really great solvent whenever you want to have ions around. The insoluble product compound is called the precipitate. Double replacement reactions always occur in water, with the reactants in the aqueous state. Luckily, there aren't that many strong acids and bases, and you can learn morem about this from this video: Īnything that is soluble in water and dissolved (separated into individual cations and anions) is in the aqueous state. It is helpful to have the strong acids and bases memorized, since they have special reactivity. The cation (or positively charged ion) of the salt comes from the base, and the anion (or negatively charged ion) comes from the acid. If you have tried this reaction at home, you probably remember a lot of fizzing because the neutralization reaction is accompanied by a gas-producing reaction, where the carbonic acid decomposes into carbon dioxide gas-bubbles!-and water.Ī salt is generally any ionic compound, though I have also seen it defined as an ionic compound that is formed when you react an acid and a base. A + B − + C + D − → A + D − + C + B − \greenD NaCH 3 COO start text, N, a, C, H, end text, start subscript, 3, end subscript, start text, C, O, O, end text.


 0 kommentar(er)
0 kommentar(er)
